| THERMO Spoken Here! ~ J. Pohl © | TOC NEXT ~ 193 |
Yardley's Extractor (1856)
This introductory writing about thermodynamics is restricted. To present the full use of water in industrial applications (at its varying and much higher pressures) would require pages beyond our scope. However, one high-pressure example is presented for those students who boil bones to make glue.
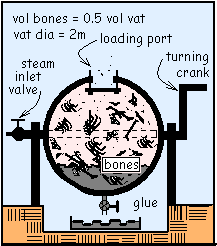
Yardley used a spherical vat with hot high-pressure steam to render animal bones into glue. The vat was filled with bones, fat and cartilage. Steam was admitted to purge the air, then the vat port cover was pulled snug. Next the vat was charged with steam to 250 kPa and 500°C then rotated slowly. It usually took about an hour for the steam to cool until the pressure in the vat became one atmosphere whereupon the port cover would pop open. Then the glue was drained into the sump and the process was repeated. The first time this cycle is performed nearly none of the bone dissolves so the "glue" is nearly pure water. Data for water are given with the p-v sketch.
What mass of steam is required to fill the extractor?
♦ The mass of steam is simply the extractor's volume divided by the specific volume of steam.
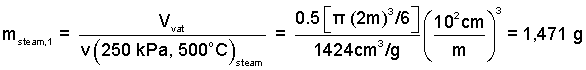
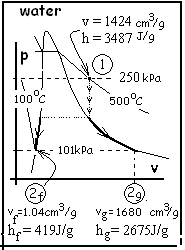
Calculate the amount of liquid when the port pops open. In the second state, the original mass will be part liquid with the specific volume of state (2f), and part vapor with the specific volume, of
state (2g). Equations for the sums of their masses and volumes are:
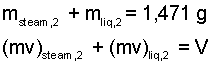
Making the equation immediately above explicit, we have:
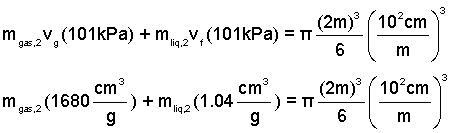
Now we have two equations with two unknowns:
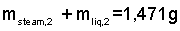
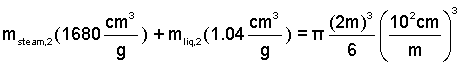
By solution of these equations, we determine the mass of the liquid to be:
mliq,2 = 1238 g
Calculate the average rate of cooling of the vat. Yardley was thinking about air-conditioning for the vat room. The a/c unit would have to match the cooling of the steam in the vat which happened in about one hour. The steam has negligible kinetic and potential energy changes. And since its volume is constant, there is no work. All of the heat passes to the surrounding ambient air. Thus our energy equation is:
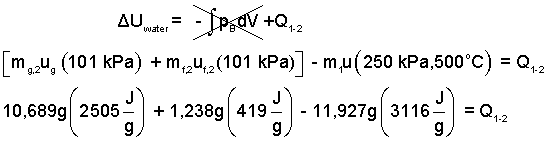
Calculation of the vat cooling (negative heating) rate is as follows.
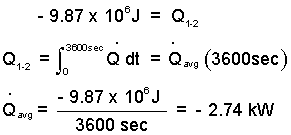
The negative sign means the heat transferred from the steam to the surroundings (as we expected).
Yardley's Extractor (1856)
This introductory writing about thermodynamics is restricted. To present the full use of water in industrial applications (at its varying and much higher pressures) would require pages beyond our scope. However, one high-pressure example is presented for those students who boil bones to make glue.

Yardley used a spherical vat with hot high-pressure steam to render animal bones into glue. The vat was filled with bones, fat and cartilage. Steam was admitted to purge the air, then the vat port cover was pulled snug. The vat was charged with steam to 250 kPa and 500°C then rotated slowly. It usually took about an hour for the steam to cool until the pressure in the vat became one atmosphere whereupon the port cover would pop open. Then the glue was drained into the sump and the process was repeated.