| THERMO Spoken Here! ~ J. Pohl © | TOC NEXT ~ 186 |
Chef Thickens the Soup

A pot on a range contains four liters of soup at a gentle boil. The chef intends to thickened the soup, slowly, by boiling away 30% of its water. The heating element of the stove is rated at 1800 Watts.
Estimate the least time of heating required to thicken the soup.
♦ We select the 4 liters of water as a closed system, Fig-1 shows the soup as our syatem, initially and finally.
Some call this type event a "batch process." The event "commences" and at a later time attains its second condition. Although good soup is not water, we approximate the soup to have the properties of pure water. In this case the soup, initially, is boiling. Then continues to boil until 30% of the system (water) has changed to vapor. As water heats to vapor it expands into the kitchen.
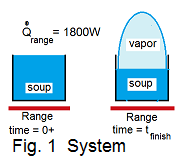
The immediate surroundings, the air within the kitchen, is at atmospheric pressure and the boinling will not increase that pressure. Hence the heat of the "thickening" process of the water occurs at the constant pressure of the surrounding atmosphere. Upon boiling, the water (initally and water-plus-vapor, finally) will experience near zero changes of kinetic and/or potential energies.
However the boiled water will experience a change of volume. Work happens by virtue of the expansion of part of the water - as part becomes vapor and "backs-off" the surrounding air at one atmosphere.
A basic energy equation, written in rate-form is:
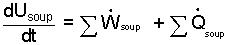
| (1)
The subscript "soup" identifies the system. Summation signs, Σ are use with "work" and "heat" as a reminder to look for all instances of either. |
The "work" and "heat" of Eqn-1 are written preceded by "summation sign" to remind us to address terms carefully. All substances are at least "simple compressible." "Other work" for the soup does not apply. Compression work (rate-form) is written as below-right.
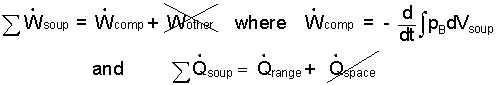
| (2) |
Expanded summations of (1) are:
Above, the "heat" of the soup some that "arrives" from the range some that "leaves" passing from the boiling water into the surrounding kitchen space/air. At our level of study, the heat from the water to the kitchen cannot be calculated. Consequently we must assume this amount of heat is "small or zero;" we strike it out (/). Yet by neglecting this heat (which is not zero) we realize the answer we obtain will be the "minimum" time for the event. Our energy equation is:

| (3) |
This is a first-order ordinary differential equation. The next step tpward solution is to separate variables.
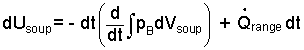
| (4) |
4
By definitions of calculus, the compression work term reduces to be the boundary pressure times differential volume. Also the boundary pressure of the soup equals the ambient pressure of the air.
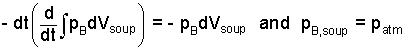
| (5) |
5
Some algebra along with the definition of enthalpy (h = u + pv) yield a final differential equation form.
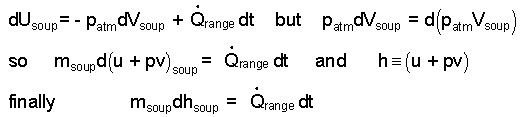
| (6) |
6
Next apply the linear integration operator to the equation. The limits of the integral "left-of-equality" are the initial and final state of enthalpy of the water. Right-of-equality the limits of integration are the corresponding times, commencement of heating and the "unknown," time of completion of the event.
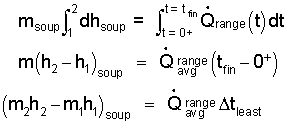
| (7) |
7
To proceed we must cast the initial and final enthalpies in terms their masses and specific enthalpies. The initial state is saturated liquid at one atmosphere. In the final state, some water remains as saturated liquid and some has "boiled-off" as saturated vapor. The subscripts " f " and " g " are used for the saturated liquid and vapor states, respectively. Some algebra is requires:
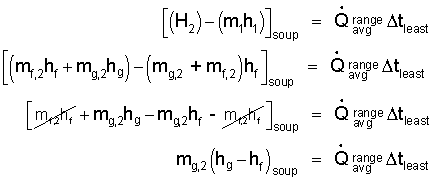
| (8) |
8
Thirty percent of the original mass of saturated liquid is heated to become vapor. Thus the mass of vapor generated is:
| mg,2 = 0.30 [4000 cm3(1 g/cm3] = 1200 grams. | (9)9 |
The enthalpy of phase change (some call this "latent heat of caporization at one atmosphere") is:
| hg - hf = (2675 - 419) J/g | (10)10 |
Entering these numbers we obtain the "least" time required.
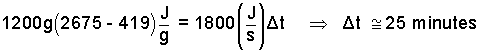 | (11)11 |
Thermodynamic calculations do not provide correct answers. Only approximate answers are obtained. In this case this time is the least because we realize some of the heat of the cooking range will pass to the surroundings.
Chef Thickens the Soup.

A pot on a range contains four liters of soup and is at a gentle boil. The chef intends that the soup be thickened slowly by boiling away 30% of its water. The rating of the stove element is 1800 watts.
What is the least time required to thicken the soup?
Premise presently unwritted!