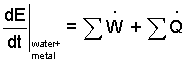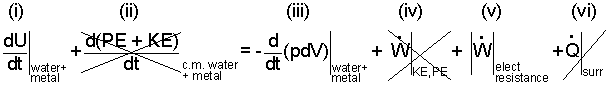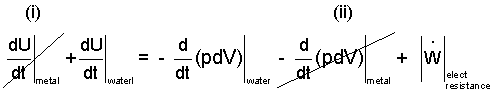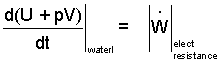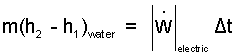| THERMO Spoken Here! ~ J. Pohl © |
TOC
NEXT ~
187 |
When will the Teapot Whistle?
Suppose you pour one liter of water into a thermally insulated, electrically heated teapot then you turn it on. The label on the teapot states that in operation, it draws 1250 Watts of power.
 The metal of the teapot is "thermal equivalent" to 200 cm³ of liquid water.
The metal of the teapot is "thermal equivalent" to 200 cm³ of liquid water.
i) What is the very shortest time you must wait before the water boils and the pot begins to whistle?
ii) Assume you get so involved in your thermo homework that you forget to pull the plug on the kettle, once started, for how long will whistling of the teapot continue?
Discussion and Solution: We take the system to be the water and the pot - its metal. We begin by writing the energy equation in a first, implicit, or skeletal form. There are three terms. At any instance in time, the rate change of energy (of the water and teapot) equals the sum of work rates applicable plus the sum of heat rates applicable. The implicit equation for these materials as they sit on the table and as they operate (once initiated) is:
Equation (1) is expanded to become Eqn-2.
(i) represents the rate of change of the internal energy of the pot materials and of the water it contains.
(ii) represents the "possibility" of kinetic and/or potential energy change of the center of mass of the water or teapot materials. These are zero, of course; the pot
does not move, the pot whistles with the first water that boils; that water moves slowly.
(iii) Work attends events for which a system (or its components) expands (or contracts). The water experiences expansion. The pot does also but only very slightly.
(iv) this term represents whatever work might have occurred that caused a change of kinetic or potential energy of the water and/or pot. For this case this is zero.
(v) one way to treat electrical resistance heating is to consider it to be "frictional" electric work of the system.
(vi) as heating of the water and pot occur, the temperature increase of
the substances (above their initial ambient temperature) will provide an incentive for heat from the system to the surroundings. This, being very hard to calculate, is ignored with first calculations (and included later if necessary).
We arrive at a new equation:
By striking (i) and (ii) in the above equation to be zero, we have approximated the teapot as being only the water. The "frictional electric work" is delivered to the water while the teapot experiences no change.
The remaining term, (3) left-of-equality, and the first term, right-of-equality, combine to be enthalpy for water.