| THERMO Spoken Here! ~ J. Pohl © | TOC NEXT ~ 177 |
The Complex Substance
Analytic methods of physics and engineering address the real world only in a vicarious sense. The real world, being complex, is studied "approximately" in terms of models. BODY, is the simplest model, substance (or pure substance) is common. The matter of some systems qualify as Complex Substances.
Substance: The basic model of thermodynamics is termed "substance." A principal assumption of the model is that the system mass consists of a single chemical species that is pure, not a mixture. Some use the term "pure substance." Nothing is pure, nonetheless, thermodynamics begins its basic experiments using matter as pure as possible or in analysis, the matter is assumed 100% pure.
Thermodynamics, an extension of physics, defines the properties (internal energy and temperature) and system energy transfer mechanisms as heat and work then explores their inter-relations for thermodynamic events.
Thermodynamic work involves change of thermodynamic properties of the system substance. Each substances has an inherent thermodynamic "force-like property" and a "displacement-like property." In a generalized way when "force acts through displacement" substance energy changes via work. Special types of thermodynamic work are known. Each distinct type is called a work mode.
All thermodynamic substances have one work mode which is compression work. This is to say the energy of all substances can be changed by its compression. Such work can be put to use for a gas as system but rarely for liquid or solid substances. The list of work modes (all thermodynamic) is: compression work, spring work, torsion work, surface work, electric work, electric polarization work, and magnetic polarization work. A substance with one (and only one) work mode is termed "simple."
Complex Substances: To great utility of engineers are the few exotic substances that have two significantly appreciable work modes. Such a substance might experience work in its first mode then immediately react by work in its second mode. The input/output nature has proven very useful. Quite a few designs have sprung from such unique substances. Internet resources have details. You will need to "look around" in accord with your interest. Key words might be: Piezoelectric Sensor, Magnetostrictive Positioning, Oxygen Sensor.
Complex Substance Feasibility: Certainly, were one to apply an enormous magnetic field to a substance, one would expect it to do something, levitate or at least, smoke. Extreme values of the force or displacement properties are not useful. Value of a complex substance is realized not in the extreme but in "being complex with within subtle, non-destructive, ranges" of its two force and two displacement properties. In looking for, evaluating potential of a substance - understanding of property ranges is important. Two example investigations are below. The first is numeric, the second algebraic.
================
Example 1: Complex "Elastic/Compression" Substance: As an elastic substance is strained along the axis of extension concurrent strain happens prependicular ("perp") to the axis. Poisson's ratio quantifies this effect. Taken in combination, the strains amount to a decreased volume of the substance. Volume change of a substance within the surroundings (assumed ambient atmosphere) constitutes substance compression work. Compare these work effects. Most persons know the elastic work vastly overshadows the concurrent compression work. But how to show this.
Explanation: Strained along an axis a solid exhibits strain in perpendicular axes also. The relationship between axial and perpendicular strains, ε, for a linear Hookean media involves Poisson's Ratio, μ, as εperp = -με (μ ≤ 1/2)
The minus sign indicates that a circular member loaded in tension will be reduced in diameter. For the case, μ = 1/2, the system volume is constant otherwise the volume increases. This gives rise to compression work, pdV, accompanying the elastic work.
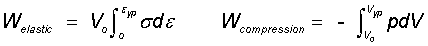
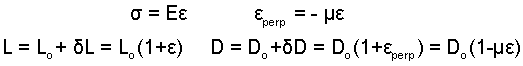

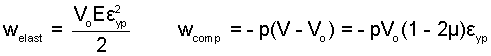
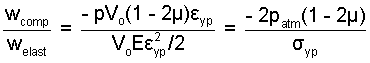
The above algebraic result is applied to results of a previous calculation: Linear-Elastic Substance.
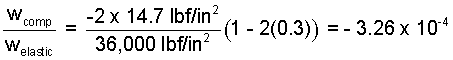
The example simply shows the procedure. As expected, the elastic work component is much greater than the compression component.
==========
Example 2: Complex "Surface/Compression" Substance: The phenomenon of boiling is critical to design of steam power production, water-moderated nuclear reactors, phase-changing refrigeration systems, cooking and other applications.
Boiling occurs when heat driven by high temperature becomes sufficient to locally vaporize a liquid. The vapor bubble is formed then grows within the surrounding liquid. Boiling involves at least two work mechanisms: surface work of the enlarging liquid-vapor interface and compression work of the expanding bubble. An algebraic expression of the ratio of these work effects is derived below.

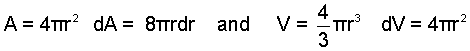
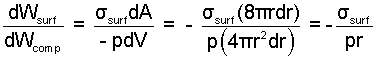
Thus the relevance of surface to compression work depends upon the radius of the bubble. Work of an infinitesimally small bubble will be dominantly surface work in nature. Large bubbles will have compression ( -pdV ) as their work form. At some "transitional radius" the work effects would be equal. For water boiling at 1 atm (σsurf = 58dyne/cm) the transitional radius, rt is:
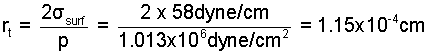
Complex Substances
Most of
Premise presently unwritten!