| THERMO Spoken Here! ~ J. Pohl © | TOC NEXT ~ 146 |
3.12 Constant Pressure: IG
Since many events occur at constant pressure (especially the pressure, one atmosphere) it is convenient to reduce the Energy Equation: SUBSTANCE to apply to that case. The beginning equation is:
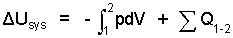
| (1) 1 |
Heating proceeds slowly such that pB equals p which is constant. Thus we can write pdV as d(pV).
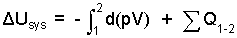
| (2) 2 |
The integration proceeds promptly and the grouping, U + pV arises and is defined to be enthalpy, H. (Image is lost)

| (3) 3 |
This result applies in general for constant pressure events of compressible substances. Enthalpy appears here and again later in a natural way. The combination of previous properties is so convenient, it is made quantitative and listed in tables. Enthalpy is: h ≡ u + pv.
Specific Heats of Solids and Liquids Being virtually incompressible, the energies of (non-exotic) solids or liquids are little changed by work. Thus their enthalpy and internal energy changes are nearly equal. Their energies are thermal, characterized by temperature with heat as the energy transfer mechanism. Thermal energy change of condensed phases is expressed as the product of mass times its specific heat (experimentally determined) times the event temperature change. In application, a constant, average specific heat is assumed, retrieved from a table and used in preliminary calculations.
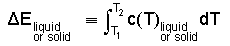
| (4) 4 |
Although specific heats vary with temperature, the usual calculation procedure is to avoid that complexity (initially, at least) by using the mean value theorem of calculus.
The following common equations (repeated from physics) are for use with single-phase solid or liquid systems:
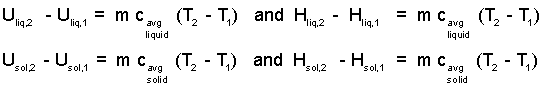
| (5) 5 |
Constant Pressure Events
Since many events occur at constant pressure (especially the pressure, one atmosphere) it is convenient to reduce the Energy Equation: SUBSTANCE to apply to that case. The beginning equation is:
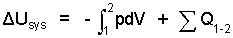
| (1) 1 |
Premise presently unwritted!