| THERMO Spoken Here! ~ J. Pohl © | TOC NEXT ~ 1 |
3.14 Frictionless Adiabatic: IDEAL GAS
Some authors use the terminology “reversible” adiabatic. “Frictionless”is better for us.
The system of this development is an Ideal Gas (IG). The IG, having its simple Equation of State, permits the math to go smoothly. There are two idealizations of the event - frictionles ans adiabatic.
Adiabatic: The idealization, "adiabatic" is easier to understand. This simply means the gas experiences no heat during its event. Impossible conditions that eliminate heat are perfect insulation and or a promptness of the event (for there to be heat requires time).
Frictionless: Work occurs to an ideal gas (in a compression or expansion event) as the system in accord with gas boundary displacement and pressures at boundary locations. While work occurs at boundaries, pressures vary within within the gas (system). Pressure shock waves travel about and pressure differences occur wherever flow "corners" happens. The pressure of the gas at localities interior are not the same but attempt to become equal, to become the same pressure at all interior localities. The gas attempts to equilibrate. These "inside" activities of the gas are waste full. to the work of the event. While the gas attempts to deliver work to the surroundings, inner friction diminishes the effect. When work from surroundings to the gas is intended, friction diminishes that.
Frictional: Put otherwise, gases experience pressure variations during any event. When they attempt to attain a uniform pressure inside, the attempt produces higher temperature at the expense of pressure.
happens that occurs to the gas in relation to its pressure at and displacement at its boundary. During compression (or expansion) a gas experiences variation of pressure, possibly shock waves and pressure Our first task is to describe the idealization, "frictionless," as it applies to a gas idealized to be an IDEAL GAS for the event. The friction we discuss is that of motion of the gas. The friction occurs at the boundary because the gas over it. But also, within the gas, some parts of the gas move differently than its neighbors. So there is friction also "within the the gas."
Beginning level methods are incapable of dealing with friction. 99% of calculations of physics and thermo texts include the assumption, no friction whatsoever, anywhere. To ignore friction in moving air (our IDEAL GAS) we assume its pressure decreases as it expands, but does so without friction, meaning uniformly throughout all of the air. This means we assume all pressure at a locations on the boundary and all pressures interior are the same, pB = p, for all boundary locations and interior locations for the air for all stages of its expansion. Albeit, IG events have pressure change; we assume ALL pressures are at all times equal, one to the other - for all. This is a BIG assumption. What can be concluded by use of such a crass approximation?
Assuming a "frictionless-adiabatic" event for air assumed IG, applying the energy equation, we obtain a simple differential equation The equation and its conditions are:
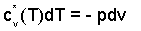
| (9)(9) |
The first step of solution is to use the gas equation to replace p with T and v. Then separate the variables and integrate.
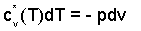
| (9)(9) |
By the rules of natural logarithms, the expression above right can be cast as a process path for the frictionless adiabatic expansion (or compression of a gas). Our immediate result has T and v as variables. Substitutions using the gas equation with a workout in dealing with exponents yield an equation form with T and p and another with p and v as variables. These are included here for completeness.
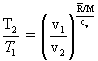
| ()() |
The left most form applies to our air pistol. Entering our numbers, the second temperature of the air is determined to be 267 K.
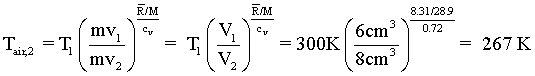 (13)(13)
(13)(13) |
It is now possible to solve for the exiting velocity of the bullet.
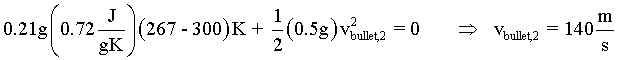
| (14)(14) |
3.14 Frictionless Adiabatic: IDEAL GAS
Some authors use the terminology “reversible” adiabatic. “Frictionless”is better for us.
The system of this development is an Ideal Gas (IG). The IG, having its simple Equation of State, permits the math to go smoothly. There are two idealizations of the event - frictionles ans adiabatic.
Premise presently unwritted!