| THERMO Spoken Here! ~ J. Pohl © | TOC NEXT ~ 106 |
Ideal Gas Equation Validity
More substances are not ideal gases than are. Thus the equation can be used "after the gas has been shown to have ideal behavior" or "by assumption that the equation applies."
Is water at STP an ideal gas?
♦ The standard pressure and temperature are: p = 101 kPa and T =25°C respectively. We are also aware that water is a liquid (not a gas) at STP and that the specific volume of the liquid is v = 1.0 cm³/g. For water to qualify as an ideal gas its properties when placed in the Ideal Gas Equation, arranged as below, must equal 1, or be very close to "1".
NOTE: The Ideal Gas Equation, written as: pv = (R/M)T is a form very well suited for use. Nearly always, to rearrange the equation yields no advancement buy only scrambles the basic form. However, every rule has its exception.
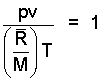
| (1) (1) |
So put the numbers in the equation and make sure the units are correct.
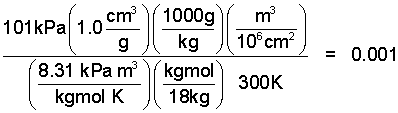 | (2) 2 |
Obviously! We knew to begin that water is not a perfect gas.
Ideal Gas Equation Validity
More substances are not ideal gases than are. Thus the ideal gas equation can be used ONLY "after the gas has been shown to have ideal behavior" or "by assumption that the equation applies." Is water at STP an ideal gas?
Premise presently unwritted!