| THERMO Spoken Here! ~ J. Pohl © | TOC NEXT ~ 107 |
Fuel Cell Proportions
A fuel-cell is a battery-like chemical device into which flows oxygen and hydrogen. These species react to produce electrical potential and current. The exhaust of the fuel cell is water. Chemical thermodynamics is a very important discipline.
To now, we have avoided involvement with moles of gases by always writing the gas constant as a quotient: R/M. Inevitably one must relate moles to mass and vice versa. To that purpose we include this simple illustration that mass is "conserved" in the (simple, complete) hydrogen and oxygen reaction that forms water. Similar approaches might be used elsewhere.
In a fuel cell, hydrogen is oxidized (by oxygen) in the following ideal reaction of moles (numbers of particles) of each type (species) of matter:
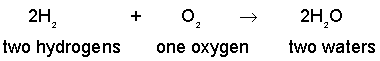 |
(1)1 |
The equation implies a perfect and complete reaction in which two molecules of hydrogen
(M = 2.0) react with one oxygen molecule (M = 32) to produce 2 water molecules (M = 18).
What about the masses of the complete oxidation reaction of a mass of two kilograms of hydrogen? What mass of oxygen is needed and what mass of water will be produced?
♦ Multiply the number of moles of each species by its molecular weight, M. Since m = ηM, this will change the equation to a mass basis. The units of M are kg/kgmol.
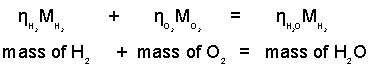 |
(2) (2) |
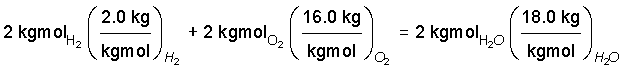 |
(3) (3) |
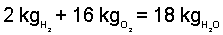 |
(4) (4) |
So 16 kilograms of oxygen are needed and 18 kilograms of water will result.
Fuel Cell Proportions
A fuel-cell is a battery-like chemical device into which flows oxygen and hydrogen. In the fuel cell these species react to produce electrical potential and current. The exhaust of the fuel cell is water. As one comes to understand basic thermodynamics, one is obliged to learn some basic chemistry.
Premise presently unwritted!