| THERMO Spoken Here! ~ J. Pohl © | TOC NEXT ~ 94 |
Ideal Gas Calculations
It is best to show a little discipline when using the
Ideal Gas Equation. The equation involves 5 entities
with both mechanical and thermal units. Is there a
best (least headaches) way for a beginner to use the
equation? Yes!
The specific energy of a single phase state of a pure substance is least as solid, greater as liquid and greatest as a gas. In general, the greater the temperature or lower the pressure (or both trends combined) the more likely a gas is to be Ideal whereupon the pressure, volume, mass, gas constant, molecular weight and temperature are related as:
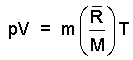 |
(1)
This form shows the equivalence of mechanical energy
(pV) and thermal energy [m(R/M)]T. |
Eqn-1 includes the Gas Constant, R, and the gas molecular weight, M, written as a quotient... (R/M). To learn the number and units of the Gas Constant and the numerical value of the molecular weight is superior to knowing the divided result.
The form of Eqn-1 is extensive. That is the equation applies to a system for which mass and volume, the extensive properties pertain. Sometimes analysis will require only intensive properties. In such cases, divide Eqn-1 by the mass and use the definitions of specific volume, v and its reciprocal, density, ρ.
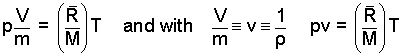 | (2) (2) |
In usual applications the gas species is known, hence its molecular weight is known and the Gas Constant is a constant, of course. Thus given values for V, m and T, it is an easy matter to solve the above equation (as is) to determine the pressure of the gaseous state. For example:
Calculate the pressure of one kilogram of air at 300 K. The mass of the gas is 1.17 kilogram and it is contained in a space of volume one meter cubed.
♦ Air is a homogeneous mixture. (Air is the ONLY mixture addressed by basic thermodynamics. All other ideal gas substances are assumed to be pure substances.) The effective molecular weight of air, Mair, equals 28.9.
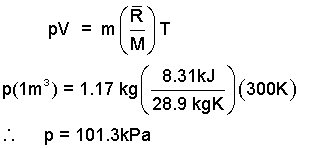
| (3)
This equation is clear. Learn to use it AS IS. Algebraic manipulation brings no meaning; it only scrambles the units. |
So we apply the numbers to Eqn-1 as it is. The calculation is easy to do on the calculator and using the above units kJ, kg, K, and m3 we know the unit of the resulting pressure is kilo-Pascals. Develop the habit of leaving equations alone.
However, what happens as the first step is most students perform an algebraic manipulation to recast pV = m(R/M)T as equation a) below for solution for pressure or as b) or as c) or d) to solve for V, m , or T respectively.
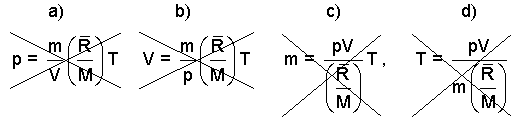 |
(4)The Ideal Gas equation does not need to be “re-arranged”. To re-arrange is a waste of time that scrambles the units making them harder to deal with. The mass, momentum, and energy equations are physical ~ they should not be “re-arranged”. Whoops, notice the algebra error I made c). Avoid useless algebra! |
Resist the impulse to rearrange the equation, pV = m(R/M)T, according to the goal of algebra, "Get the unknown left of equality." The equations a) through d) contain no information not contained in the original form (notice one, some (?) are wrong!). Learn the proper units for the original arrangement then use it and them.
Other equations of thermodynamics (as part of their formulations) are physical as well as algebraic. In those equations, terms left of equality are there for a physical reason and terms right of equality are there for some other reasons. AVOID algebra until all terms of an equation are numbers except one - then solve.
Ideal Gas Calculations
The specific energy of a single phase state of a pure substance is least as solid, greater as liquid and greatest as a gas. In general, the greater the temperature or lower the pressure (or both trends combined) the more likely a gas is to be Ideal. Although we discuss the first, extensive form of the ideal gas equation, the results apply to either form.
Premise presently unwritted!