| THERMO Spoken Here! ~ J. Pohl © | TOC NEXT ~ 72 |
Pascal's Experiment

The year after Torricelli died (1648), Blaise Pascal convinced his brother-in-law, Perier, to conduct a test of a mercury barometer by carrying one from the base to the top of the French volcanic formation, Puy de Dome. "Tell me," Blaise asked, "What difference in mercury height you observe." The density of atmospheric air is about 0.075 pounds per cubic feet. The density of mercury is close to 13.6 times the density of water. The peak of Puy de Dome is 4803 feet above its base.
Approximately what difference in heights of mercury in the barometer would Perier have observed?
♦ The sketches show the barometer readings as Perier would have observed them. The pressure in the top of a barometer, in the seemingly empty space above the column of mercury, is not zero; at 25°C it is about 20 Pascals. But that pressure is so small that it is usually approximated as zero.
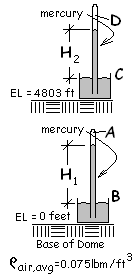 The hydrostatic equation relates pressure changes with elevation change and fluid density along a selected path through the fluids. The path we will follow begins at point A in the mercury vapor at the top of the barometer at ground level. The pressure there is about 20 Pa.
The hydrostatic equation relates pressure changes with elevation change and fluid density along a selected path through the fluids. The path we will follow begins at point A in the mercury vapor at the top of the barometer at ground level. The pressure there is about 20 Pa.
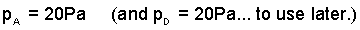
|
(1)
Pressure in the "void" at "A". |
We begin at "A" in the barometer which rests on Earth at the base of the volcanic formation. From the near zero value of pressure in the void of this barometer, pressure in mercury increases at points along a path passing downward to the mercury/air interface, "B." The pressure at all locations "B" (in mercury) at the air/mercury interface is "pB."
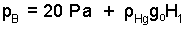
| (2)pressure in mercury |
Our next task is to estimate the pressure change associated with change of position (in air) from the base to the top of the volcano. Pressure changes along that path and so does density. The relation we have is:
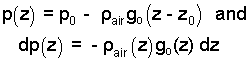
| (3)3 |
The pressure difference of our interest is pC - pB. To obtain this we integrate the differential of pressure from pB to pC.
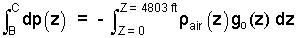
| (4)4 |
We see the integral requires that we know how the density of air and the local acceleration vary over the elevation change. We know gravity changes very little. For the air, we assume density is constant at an approximated surface value of 0.075 lbm/ft3. Thus, in effect, we use the Mean Value of Calculus to perform the integration.

| (5)5 |
The logic that applied to the barometer at ground level is applied to the barometer on top.
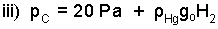
| (6)6 |
We can combine i), ii), and iii) to determine: H1 - H2
There is an easier way to solve hydrostatics problems. We recognize the two known pressures (at the top of the barometers). So we write one equation combining the pressure differences.
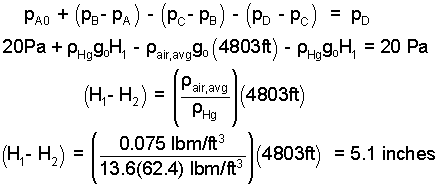
| (7)7 |
Observe: Pressure at A increased by the column of mercury (going down, therefore "+") decreased by the column of air (going up "-") decreased by column of mercury (going up "-") equals pressure at D.
Pascal's Experiment

In 1648, the brother-in-law of Blaise Pascal carried a mercury barometer from the base to the top of the French volcanic formation, Puy de Dome. The peak of Puy de Dome is 4803 feet above its base.
Approximately what difference in heights of mercury in the barometer were observed?
Premise presently unwritted!