| THERMO Spoken Here! ~ J. Pohl © | TOC NEXT ~ 241 |
Tomato Juice
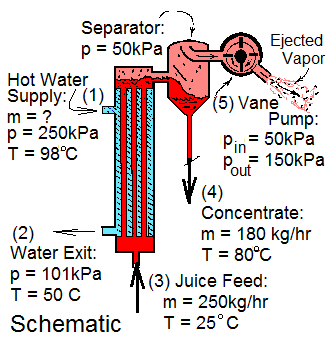 Tomatoes and tomato juice are easily damaged by high temperature. Concentration of tomato juice is accomplished using modest heating (by hot water in a heat exchanger) followed by mechanical extraction of water by action of a vane pump. The vane pump maintains a low pressure over the juice and ejects water as vapor from the separator.
Tomatoes and tomato juice are easily damaged by high temperature. Concentration of tomato juice is accomplished using modest heating (by hot water in a heat exchanger) followed by mechanical extraction of water by action of a vane pump. The vane pump maintains a low pressure over the juice and ejects water as vapor from the separator.
The concentration process has two flow streams. Hot water (1) enters, cools as it passes the juice (in counterflow) then exits (2). The other stream, juice, enters, (3) is warmed then enters the separator where its stream divides into concentrate exiting as liquid (4) and vapor exhausted by the vane pump (5). Use the information of the drawing.
Calculate the mass rate of water that exits the vane pump as water vapor (5).
♦ By the Schematic, we visualize the system as juice which enters ar (3) at 250 kg/hr and exits as concentrate at 180 kg/hr and as vapor ?kg/hr. The mass equation for steady production of concentrate is:
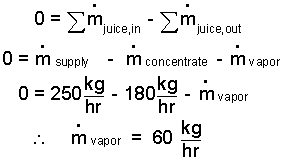
| (1) 1 |
Calculate the mass rate of Hot Water for this system.
♦ Take the heat exchanger as system and assume it is frictionless and adiabatic. The event is steady and there is no work. The energy equation is:
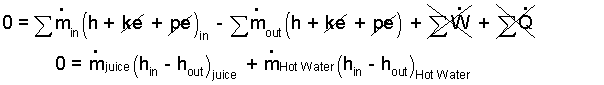
| (2)2 |
We approximate the juice as water. They are liquids as they interact in the heat exchanger. We use specific heats to express the enthalpy changes.
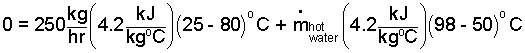
| (3) 3 |
Solving Eqn-3, we obtain:
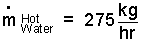
| (4) 4 |
Calculate the least horsepower of the vane pump.
♦ We write the energy equation for steady operation of the vane pump. The kinetic and potential energy changes of the flow of vapor through the pump are negligible. The vapor passes through the pump quickly with too little time for heat.

| (5) 5 |
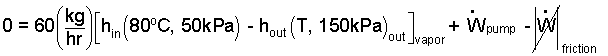
| (6) 6 |
Eqn-6 contains three unknown quantities which are the exiting temperature of the vapor, the work rate of compression and the friction loss work rate. To continue in solving Eqn-6 we require another condition or relation for the process. That condition is that the vapor proceeds along an adiabatic-frictionless path. Water vapor at this low pressure can be approximated as an ideal gas with specific heats and a specific heat ratio of:
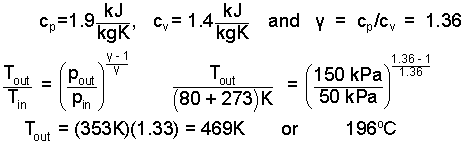
| (7) 7 |
We return to the energy equation with this information.
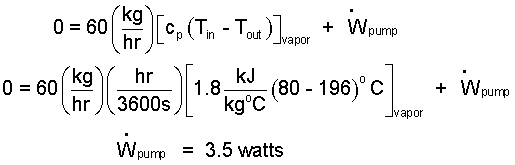
| (8) 8 |
A style of this author is to avoid algebra. The positions of the terms of an equation are part of the meaning of the term. Nothing is gained by rearranging a thermodynamic equation.
Tomato Juice

This juice is easily damaged by high temperature. Consequently its concentration is accomplished with modest heating by hot water in a heat exchanger followed by extraction of water from the juice by action of a vane pump that maintains a low pressure over the juice and ejects water vapor from it in a separator.
The process has two flow streams. The hot water simply enters (is cooled as the juice is heated) and exits. The other stream, juice, enters, is warmed then exits partly as concentrate and partly as vapor exhausted by the vane pump. Use the information of the drawing.
Calculate the mass rate of water that exits through the vane pump as water vapor.
Premise presently unwritted!