| THERMO Spoken Here! ~ J. Pohl © | TOC NEXT ~ 239 |
Water Seeks its Level
The waters of Earth exist predominantly as liquid. Wherever liquid water is found, it resides with its surface locally flat and tangential to Earth. Furthermore the surface is as physically low, that is, as close to Earth as possible - subject to constraints that contain it.
In this example we consider an amount of water initially constrained within a tank. The "event" occurs when the initial constraint is removed. The water, once freed from constraint moves toward Earth and continues to move until it meets some constraint to its flow. With no constraint, the water move, however slowly, to become part of the sea.
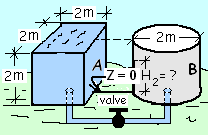 There are two water tanks connected by
There are two water tanks connected bya pipe with a closed valve. One tank is full,
the other is empty. The valve constrains the water.
The sketch depicts two steel tanks connected by a pipe with a valve. The valve, our event catalyst, is closed and the tanks are open at their tops. Tank A is completely full of water and tank B is empty.
What Second Depths result in the tanks once the valve is opened and left open a long time.
♦ The numerical answer to this physical event can be obtained by most students without thermo, even without using a plan. Numbers and answers, of course, are of less value than understanding. Physical situations of this text (like these tanks) are presented for the purpose of teaching the plan and method by which answers are obtained. This discussion (and those that follow) will show how the definitions, ideas and methods of thermodynamics constitute a plan.
Select a System (and boundary): As system, we imagine all of the water to be selected as the system. The system boundary is the outer surface of the water (its surface of contact with the air above and elsewhere where it contacts the steel of the constraining tank).
Describe and Sketch the Event: The event is an incremental change of the water from an initial equilibrium state (1) to a final equilibrium state (2). The event is initiated (t = 0+) when the valve is opened and water commences to flow from tank A to tank B. The event is defined to end with water in both tanks to depths, HA,2 and HB,2 (measured from the respective tank bottoms which are level). A sketch might be drawn identifying the system configurations (initial and final). The sketch we have will suffice.
What are the physical constraints? Initially the water is in gravitational equilibrium with Earth, subject to the structural constraint of the steel of tank A and the closed valve. In the final state, water is again in gravitational equilibrium with Earth subject to constraints of the tanks connected.
Apply the Property Equations:
Mass Equation: Thermodynamics describes the mass of this event using a notation from calculus:
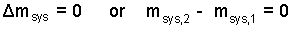
| (1) 1 |
Initially the water occupies only tank A and tank B is empty. Finally there is water in both tanks. The mass equation in difference form is:
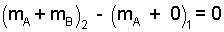
| (2)2 |
The water masses can be factored as density times volume: m = ρV.
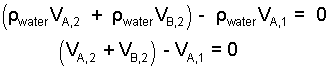
| (3) 3 |
The density of water is uniform, permitting the equation to be divided by density. Next we write the tank volumes in accord with their geometries and water depths: A being square, and B, cylindrical.
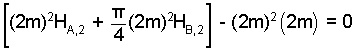
| (4)4 |
We run a check. Our steps and calculations above are correct. It is tempting to set HA,2 = HB,2 in the above equation. We know that is the case.
It is Earth, water (and the valve) that decide "what happens." For thousands of years water has been observed to "seeks its level." We argue that Earth's gravity causes contiguous water (released from constraints) to attain one surface. Setting the above depths equal, the final depth of the water (above tank bottoms) is:
H2 = 1.12 m.
Momentum Equation: The time or duration of our event is long. Momentum is initially and finally zero.
Energy Equation: What does the energy equation tell about this event? Potential energy of a body (or point mass) equals its mass times local gravity times its elevation relative to a selected datum for zero potential energy. Our selected datum is notated in the sketch, Z* = 0 . The water is not a body; it is a uniformly distributed fluid mass. Its potential energy can be expressed in terms of its center of mass (c.m.) can be used. Being uniformly distributed, its center of mass and its "center in space," have the same elevation.
PEwater,1 = mgo(Zc.m.,1 - Z*) = mgo(1 - 0)m
Potential energy is additive. After the event, the potential energies become:
PEwater,2 = mgo(Z2,c.m. - Z*)
Thus,
ΔPEwater = PEwater,2 - PEwater,1 = mgo[(Zc.m.,2 - Z*) - (Zc.m.,1) - Z*)] or
ΔPEwater = PEwater,2 - PEwater,1 = mgo(Zc.m.,2 - Zc.m.,1)
This equation shows that the datum taken of potential energy equal to zero (Z* = 0) is irrelevant. Next the tank geometries are regular. Initially the center of mass of the water is one meter above the bottom of tank A (or half of 2 meters). Later, (as calculated above) the water depth above the bottom in both tanks is 1.12 m. Thus, the second elevation of the water center of mass is: Z = (1.12/2)m or 0.56 m.
ΔPEwater = ρVgo(0.56m - 1.0m) c.m.,water
ΔPEwater = 1000 kg/m3 (8 m3)(9.81 m/s2)(0.58 m - 1.0 m)c. m,water
ΔPEwater = - 34,531 J
What became of this -34,531 Joules? Since mass moved above earth from a higher elevation (and PE) to a lower elevation (and Pe) we know ΔPE (i.e., PE2 - PE 1) must be negative. (Had the number been positive, we would know something was wrong). During the event, as water flowed, friction (mechanical dissipation) occurred. The friction caused the internal energy of the water to increase, evidenced by a slight increase of water temperature. Basic physics permits us to calculate the maximum possible temperature increase. The energy of the water is initially potential energy. The water retains that energy as it "seeks its level." The energy form changes from potential to internal No energy is "lost," the energy equation is:
ΔU + ΔPEwater = 0 or
mc(T2 - T1)water - 34,500J = 0
The specific heat of water is: cwater = 4.2 kJ/(kg °C).
1000 kg/m3(8 m3)[4.2 kJ/(kg °C)](T2 - T1) - 34,531 J = 0.
(T2 - T1)water ~ 1 °C
The initial temperature of the water was the same as the surrounding atmosphere. We assumed it to be 25°C. We determined the second temperature of the water to be a maximum of one degree greater. With time, since temperatures equilibrate, this thermal energy of the water will distribute by the mechanism of heat to the surroundings until the temperature of the water in the tanks returns to 25°C. In conclusion, when water "seeks its level," the temperature of the universe increases (very very slightly).
If parts of the solution above seem pedantic, the cause might be that you "knew the answer" to begin with. Here, method is stressed. Solution of more difficult problems requires a diligent method which, at best, yields an approximate solution or, that failing, the diligent method points specifically to what "more information" is needed for solution. Although examples and illustrations of this writing are contrived, the underlying methods of solution and the understanding therein are genuine. Numerical answers provide a concreteness to our approximated solutions.
 There are two water tanks connected by
There are two water tanks connected bya pipe with a closed valve. One tank is full,
the other is empty. The valve constrains the water.
Water Seeks its Level
The waters of Earth exist predominantly as liquid. Where liquid water is found it resides with its surface flat and tangential to Earth. Furthermore the surface is as physically low, or close to Earth as possible - subject to constraints that contain it. This example considers an amount of water initially constrained within a tank. The "event" initiates when the constraint is removed. The water, freed from the constraint flows toward Earth until a new "lesser" constraint arises. At the least constraint, water becomes part of the sea.
Premise presently unwritted!