| THERMO Spoken Here! ~ J. Pohl © | TOC NEXT ~ 202 |
Block Slides on Ice
A block of copper (m = 1.6kg) rests upon a smooth plane of ice. Initially the temperatures of the copper, the surrounding atmosphere and the ice are 0°C. In an instant, motion is imparted to the block causing it to slide across the ice slab at an initial speed of 2.5 m/s. Also immediate, sliding friction acts to cause the speed of the block to decrease. Ultimately the block comes to rest, attains the speed, 0 m/s.
Calculate the greatest mass of ice (solid water @0°C) that is changed to water (liquid water @0°C) by the event.
System: It is ice and copper that interact. We take the system to be the copper and that ice initially solid which finally is liquid (at 0°).
Energy Equation: The physics is an "increment" event. Everything is 0°C to start, and everything is "vast" so we assume all stays 0°C; hence there will be no heat. (also a "prompt" event promotes the assumption: no heat.
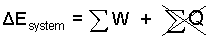
| (1)No HEAT. Everything is vast and at 0°C |
The "system" is two parts. We expand "energy" to identify its parts as "copper" and "water."

|
(2)The system is copper (assumed solid initially and finally) and water (assumed solid initially and liquid finally). |
The easiest next step is the identify the ΔE's as internal energy and kinetic energy changes.
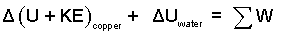
| (3) Energy equation of the event. |
A bit of thinking is involved. Both copper and water are "simple compressible" substances. The work term, ΣW, expands but it does not have a "friction" term because that effect is interior to the copper/ice event. Pressure is constant at 1 atm, the work of copper and water are as shown below.
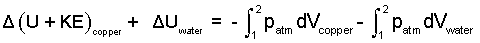
| (4)
For the event copper and water have "work" in their "pdV" forms. |
We take two steps to proceed. First we integrate the two integrals immediately right-of-equality to get their "Δ" forms.
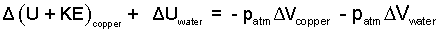
| (5) (5) |
Next we move those "work" pieces left-of-equality to right-of-equality, collect them and use the fact, "h = u + pv ".

| (6) (6) |
Apply the numbers.
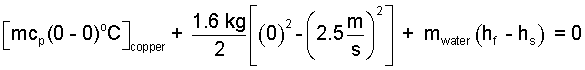
| (7)(7) |
Put the given numbers into the above. But in reality, were one to slide a copper block as depicted, would there be a small trail of liquid water left. Might one gather that water and measure its mass? No. It is unlikely that air and ice might be at precisely 0°C.
Another thought is that the copper block might simply have been dropped from a height to have the 2.5 m/s speed upon impact. The same solution as above would apply.
Copper Block Slides on Ice
A block of copper (m = 1.6 kg) rests upon a smooth plane of ice. Initially the temperatures of the copper, the surrounding atmosphere and the ice are 0°C. In an instant motion is imparted to the block causing it to slide across the ice slab at 2.5 m/s. Immediately sliding-friction causes the speed of the block to diminished; ultimately the block attains the speed, 0 m/s.
Calculate the greatest mass of ice (solid water @0°C) that is changed water (liquid water @0°C) by the event.
Premise presently unwritted!