| THERMO Spoken Here! ~ J. Pohl © | TOC NEXT ~ 191 |
Grease Fire Experiment?

A grease fire MUST NOT be combated by use of water.
When a deep-fry cooker catches fire, the usual reaction, douse with plenty of water, is a bad idea. Water thrown into flaming grease sinks beneath the grease and is heated immediately and expllosively to steam. The very great increase of volume as liquid water becomes gas, will blast flaming grease throughout the kitchen.
One morning around 2 AM two CHICKEN OUTLET new-hires bored silly, decided it would be "cool" to explode some ice in the deep-fryer.
"No flame, no problem," they assumed. As the grease in the fryer smoked gently, Frankie dropped ten grams of freezer-cold ice (- 40°C) into the vat.
Calculate the percent increase of volume of the water.
♦ Assume the ice sank immediately to the bottom of the vat and quickly became steam at 100+°C. The first panel of the table shows the path of the water from ice (1) to steam (2) on phase diagram coordinates and also on a section of the p-v-T surface for water. Immediately beneath the diagrams are listed in tabular form, normal properties for water.
| Water (in Grease) --- PROCESS AND PROPERTIES | ||||||
| p (Ts(p)) |
-40°C (sol) |
0-°C (sol) |
0+°C (liq) |
100-°C (liq) |
100+°C (gas) |
|
| 101.3 kPa (100oC) |
v~cm³/g
h~J/g |
1.08 -411 |
1.09 -333 |
1.00 0.10 |
1.04 419 |
1680 2675 |
The second volume of the ice and the percent increase of volume are:
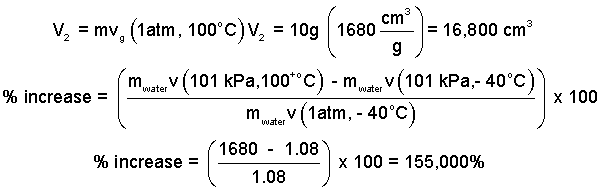
| (1) 1 |
Calculate the compression work of the ten grams of water?
♦ We would like to solve for the work directly - by performing the integration, - ∫pBdv. However, here (and usually) pB, cannot be specified. We must idealize by assuming pB equals the uniform, equilibrium pressure, patm. This makes the calculation approximate but possible.
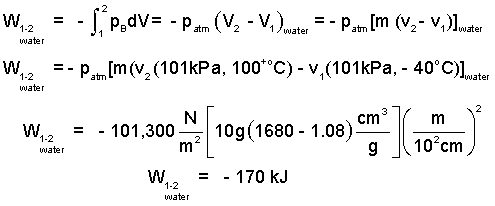
| (2) 2 |
The compression work is negative. This means that energy of the water, starting as solid and ending as vapor decreased. Does that make sense? Yes, the water was obliged to "back-off" the atmosphere; hence the negative sign.
Calculate the heat.
♦ The event of water occurs at the constant pressure, one atmosphere. There will be a kinetic energy change; we assume it is negligible. By the energy equation
for the water we have:
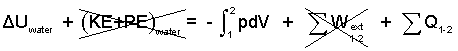
| (3) 3 |
Below, since the pressure is constant, the integration is easy. Also there is only one heat (from the grease) so we drop the summation sign. Then rearrange the equation.
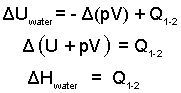
| (4) 4 |
The above table contains values for these enthalpies.
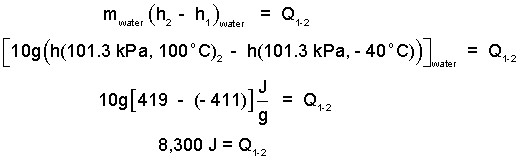
| (5) 5 |