| THERMO Spoken Here! ~ J. Pohl © | TOC NEXT ~ 148 |
3.14 Constant Volume: Ideal Gas
The usual physical scerario is an ideal gas contained in a rigid contained. Properties of the gas and container are known. Analysis proceeds by use of (i) the mass equation, (ii) the ideal gas equation, (iii) the constant volume process relation, (iv) the energy equation and (v) a specification of the gas specific heat.
The system in mind is a constant, contained mass of gas. The process is "increment" (some say "batch") in nature. Our first principle, Conservation of Mass is stated as "mass is constant" or the second and first masses of the system are equal to each other.
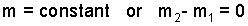
| (1) 1 |
Either we know (by having made a check), we have been told or we assume the system gas behaves ideally. This fact permits use of the ideal gas equation as a relation among properties of the system gas. The equation is written (general form) then made specific for the initial system state, (1), and the final state, (2)
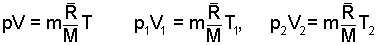
| (2)
Ideal Gas equation of state. |
For typical events, there is neither kinetic nor potential energy change. The energy equation becomes simply:
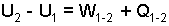
| (3)
Energy Equation (First Law) in incre- mental form, spanning an event. |
The internal energy change of an ideal gas equals its mass times specific heat times its temperature change. Work of a gas is represented as an integral which is zero for the constant volume (dV = 0) event:
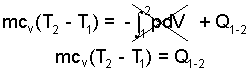
| (4)
i) Express change of internal energy as mass times specific heat times temperature change. ii) Express work as its integral. |
These are the general "ideal gas, constant volume" equations. The species of gas and its specific heat at constant volume is needed for any specific solution.
Example: A mass of air (4.64 kg) is heated at at a constant volume of 400 liters from -15°C to 195°C . Calculate the least heat required. Also determine the change of the system pressure, Δp (bar).
Heat: This calculation is straight-forward:
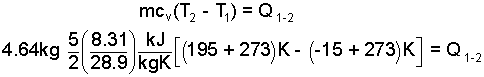
| (5)
There is sufficient information to calculate the heat of the event. |
Event Pressure Difference: There are a number of pathes of calculation. We use the, constant volume process relation with algebra to obtain Eqn-6.

| (6)
Follow through with the algebra. CHECK MY UNITS! |
The Ideal Gas Equation is used to determine p1;
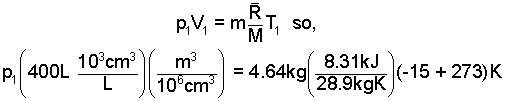
| (7)
More algebra... |
Finally, solve (7) for p1. Substitute the numerical value of p1 in to Eqn-6 (rightmost form). Write (T2/T1) as [(195 + 273)K/(-15 + 273)K]. Then run out the numbers.
Constant Volume Event: Ideal Gas
The usual physical scerario is an ideal gas contained in a rigid contained. Properties of the gas and container are known. Analysis proceeds by use of (i) the mass equation, (ii) the ideal gas equation, (iii) the constant volume process relation, (iv) the energy equation and (v) a specification of the gas specific heat.
Premise presently unwritted!