| THERMO Spoken Here! ~ J. Pohl © | TOC NEXT ~ 96 |
Casting Railcar Wheels
The somewhat elaborate apparatus is used to cast the steel wheels for railroad cars. The process begins when a large "ladle" of molten steel is placed in a
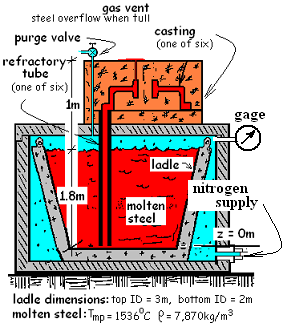 chamber which is then covered with a heavy air-tight lid. Ceramic wheel molds are arranged on top of the chamber. A "refractory tube" or pipe extends downward from each mold to the bottom of the pool of molten steel.
chamber which is then covered with a heavy air-tight lid. Ceramic wheel molds are arranged on top of the chamber. A "refractory tube" or pipe extends downward from each mold to the bottom of the pool of molten steel.
Once properly set up and sealed, casting happens as nitrogen is slowly injected into the chamber. As the pressure of the nitrogen increases the surface of the liquid steel in the ladle is displaced downward causing liquid steel to flow up the "refractory tubes" and into the wheel molds. With time, the liquid steel fills all of the molds then it fills the "gas vent" ports and ultimately, it flows to form a small puddle on top of the mold. When molten steel issues from the "gas vent," it is known that all molds have been filled. The ladle is circular with an average diameter of 2.5 meters. When filled to a depth of 1.8 meters it holds enough steel to cast 6 wheels. Use information of the sketch.
Calculate the mass of steel in a full ladle.
♦ The liquid steel contained is simply its density times its volume.
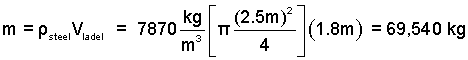
|
(1)Mass of a homogeneous substance equals its density times its volume. |
Calculate the gage reading (G.R.) of the maximum pressure the chamber must withstand?
♦ It is the pressure of the nitrogen that forces the liquid steel up the refractory into the molds.
Consequently the greatest nitrogen pressure will occur just at the end of the casting process. A reduced sketch (to the right) shows the chamber, refractory tube and mold for the condition of greatest pressure. The liquid steel is red, the nitrogen is blue.
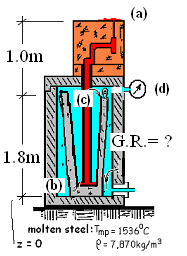 Hydrostatics of liquid steel and gaseous nitrogen are will be used to solve for the pressure.
Hydrostatics of liquid steel and gaseous nitrogen are will be used to solve for the pressure.
As with any hydrostatic situation, we begin by inspecting the arrangement with the hope of finding a place where we know (or can reasonably assume we know) the pressure. When liquid steel puddles on the tops of the molds, at the puddle locations where the steel pools. The pressure within the liquid steel will be one atmosphere at location "(a)".
Regarding the event, as liquid steel begins to puddle atop the molds (the ending moments of casting) nearly all of the steel originally in the ladle will have been pushed upward into the molds. The level of the surface of liquid steel remaining in the ladle will be quite near its bottom, at (b). And there, contacting the liquid steel, acting on it, will be the pressure of the nitrogen at its maximum for the casting event.
Our task is to write an equation that expresses pressures along a path through liquid steel. The path commences in a pool of steel that spilled onto the top of a mold, "(a)". The equation initiates with the pressure there, atmospheric pressure. The equation expressing pressure "along the path" of liquid steel will be written in increments. When the equation is complete, the last pressure will be that at (b), which is the pressure of the nitrogen at the conclusion of the casting event.
Thus the first term of our path, (1), is located at "a" in air where the pressure is atmospheric.
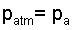
| (1) |
The first pressure is patm.
The pressure in air equals the pressure in steel at its very surface. Our path, for solution, passes downward through liquid steel to its lowest elevation in the ladle. The terms, (1)and (2), taken together, equal the pressure in liquid steel at its lowest level.
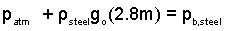
| (2)
The pressure at (b) equals patm plus the added pressure of a column of liquid steel 2.8 meters tall. |
The path of our imaginary point has arrived in liquid steel at (b). To continue, our point must move across a solid/gas interface, that is, from liquid steel to gaseous nitrogen. That pressure change, for a flat interface is zero. Thus pb,steel is equal to pb,nitrogen.
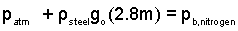
| (3)
This equation expresses that the pressure in liquid steel equals the pressure in the adjacent nitrogen. |
To continue the path of our imagined point, we proceed upward through nitrogen (however) to arrive at a location on the level of but "inside," on the nitrogen side of the gage. Assuming a constant density of the nitrogen over that elevation, the pressure decrease (term 4) to that point subtracted from the previous pressure yields:
Next, we extend the above equation by visualizing the pressure change as our point moves up 1.8 meters through nitrogen to the gage, then through the gage to the atmosphere. The correct terms added to the above yield:
Our answer is at hand, except that we don't know the density of the nitrogen. We expect the contribution of the nitrogen to be small. The temperature of the nitrogen (M = 28) is likely to equal (or less than) that of the steel ~ 1536°C. Below we write the hydrostatic equation again with the ideal gas equation for nitrogen below it.
Casting Rail Car Wheels
A somewhat elaborate apparatus is used to cast the steel wheels for railroad cars. The process begins when a large "ladle" of molten steel is placed in a chamber which is then covered with a heavy air-tight lid. Ceramic wheel molds are arranged on top of the chamber. A "refractory tube" or pipe extends downward from each mold to the bottom of the pool of molten steel. When the chamber is pressurized with gaseous nitrogen, molten steel flows from the ladle upward into the molds.
Premise presently unwritted!