| THERMO Spoken Here! ~ J. Pohl © | TOC NEXT ~ 83 |
Cave Diver
On average, 8 persons a year drown while exploring spring-fed underwater caves in Florida. In one cave a local dive club posted warning signs and installed a mercury barometer. The schematic shows a diver's view of the barometer and its location relative to the spring surface.
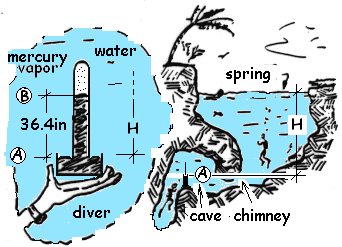
Calculate the depth of the diver (at the deepth of the barometer) beneath the surface of the spring.
♦ By inspection we know the barometer reading and the vapor pressure of mercury in the barometer (almost zero). The pressure of air at the surface is atmospheric. The depth of the diver, H, is the unknown. To solve, using the hydrostatic equation, we visualize a path through fluids (air, water and mercury) that leads from one of the known pressure to the other. Our path can start at either place but must end at the other. We write the pressure changes that occur at a point as that point is imagined to move through fluids from the spring surface to the last point, just inside the mercury vapor. We write the equation repeatedly and progressively as the terms are explained. Later such equations will be written on one line.
In air at the surface of the spring we assume logically:
| pair,surface = patm | (1) 1 |
Moving toward the mercury vapor of the barometer requires crossing the air/water interface for which there is no pressure change. Thus at the spring surface but in water the pressure is:
| pwater,surface = patm | (2) |
2
In water, we imagine our point to precede downward to the point (A) at depth H. Let the point move directly downward. As it moves downward its pressure will increase in proportion to the column of water of height, H. The new pressure at depth will be:
| pwater,depth H = patm + ρwatergo H | (3)3 |
At depth H, the "point" that traces our path must move horizontally, with no changes of pressure, to arrive just above the exposed mercury of the barometer attached to the cave wall; position, A. Next imagine the point to move downward (across the water/mercury interface) into mercury (with no pressure change) then upward in mercury and across the mercury liquid/vapor interface at the top of the barometer. (Since the liquid-to-vapor interface of the mercury is not flat there is a pressure change across the interface. This pressure change is small and negligible to this calculation). Now, once the point in the mercury vapor, the pressure of the "point" becomes that of mercury vapor, (B).
| pHg vapor = patm + ρwater gH - ρHggo(36.4/12)ft | (4) 4 |
However, pHg vapor is nearly zero. Thus,
| 0 = patm + ρwatergH - ρHggo(36.4/12)ft | (5) 5 |
Since the original sketch presented English Engineering Units are used.
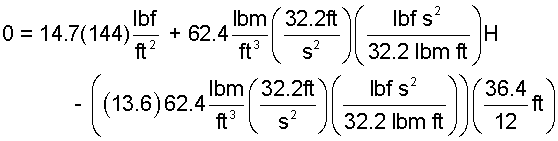
| (6) 6 |
The above is A single equation with one unknown, H.
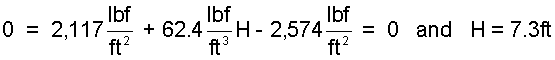
| (7) 7 |
While the above approach is clear, some persons skilled with these calculations write the equation on one line. This technique will be illustrated in the next examples.
Cave Diver

On average, 8 persons a year drown while exploring spring-fed underwater caves in Florida. In one cave a local dive club posted warning signs and installed a mercury barometer. The schematic shows a diver's view of the barometer and its location relative to the spring surface.
Calculate the depth of the diver beneath the surface of the spring.
Premise presently unwritted!