| THERMO Spoken Here! ~ J. Pohl © | TOC NEXT ~ 1 |
Concrete Mixture
Language, terms and definitions are important to physics and thermodynamics. Correct us, if you will. Does the text example: "Borgnakke, Sonntag et al ~ Example 2.1" introduce a "new" property, the "overall (average) specific volume" and "overall (average) density."
A simple classification of a mixture is: homogeneous or non-homogeneous. Homogeneous means uniform throughout. The example from Sonntag treats its mixture as being homogeneous but the figure provided by the authors shows clearly that the mixture is not uniform throughout. Indeed, since air is included as a system component, the mixture cannot possibly be uniform throughout.
Concrete is a non-homogeneous mixture. Therefore the density the authors calculate does not apply to any randomly selected volume of the system mixture. In fact, density is not calculated, whereas an "effective density" is. The text solution is shown below.
Comments:
1) The specific volume and density obtained are special - untypical. Granite, sand, water and air comprise a non-homogeneous mixture. Consequently the density calculated does not apply to any small amount of the system material selected. Here proper use of density (for the constituents) is used to obtain an erroneous answer for the mixture. Put otherwise, suppose one were to scoop 1 meter cubed of material from the container. Would one expect that mass to be 755 kg? That would not happen.
2) The authors seek "effective" specific volume (and effective density) but have not provided equations for such calculations.
This is a poor example but it can be worked more logically.
3) In problem solution it is expedient to write the "operant" equation first. When a system is composed of a number of distinct materials, the density of those materials, taken together, is called its effective density with the symbol notation ρeff and the following definition:
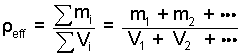 |
(1)
Effective density equals the aggregate mass divided by the aggregate volume. |
The general rule (above) applied to the constituents of the author's case yields:
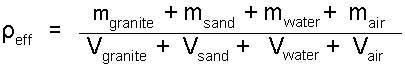 | (2) Identify the constituents. |
But the problem provides the density and volume of each constituent of the mix. So we rewrite the numerator sum:
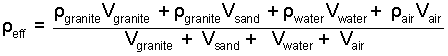 |
(3)
To complete, enter the properties of constituents and calculate. |
The authors state the problem with air "included" then remark that the air "can be disregarded." There is no need to disregard the air. Just enter its effect and the numbers it contributes. Final calculations will show the contribution of air to the solution to be small, to be negligible.
About AIR... thermodynamics treats all substances as being pure. This means water has NOTHING dissolved in it. Gold is ALL gold. However, nothing is pure. Everything is a mixture. Thermodynamics treats one substance that is a mixture as being pure. That substance is air. Although air is a mixture of nitrogen, oxygen and many other gases, it is treated as a single entity.
Concrete Mixture
At the simplest level a mixture is classified as homogeneous or non-homogeneous. Homogeneous means uniform through out. The text example discussed here (Sonntag et al. Example 2.1) treats its mixture as being homogeneous but the figure provided by the authors shows clearly that the mixture is not uniform throughout. Indeed, since air is included as a system component, the mixture cannot possibly be uniform throughout.
Premise presently unwritted!