| THERMO Spoken Here! ~ J. Pohl © ( D0050~4/15) | ( D0100 - Energy Equation - constant pressure) |
4.02 Normal Properties: WATER
The word normal means "having the pressure, one atmosphere." The brief sketch (a pressure-temperature projection of the surface of water) is called a "phase diagram." Phase change temperatures and regions of solid, liquid and gas and shown.
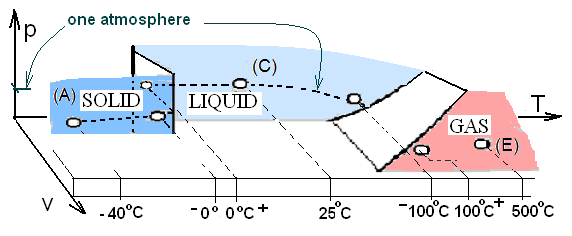
 The horizontal dashed line (pressure = 1 atm) is the locus "normal" states of water. One format for normal properties is a list of phase change temperatures, average specific heats for each phase and latent heats for phase changes
The horizontal dashed line (pressure = 1 atm) is the locus "normal" states of water. One format for normal properties is a list of phase change temperatures, average specific heats for each phase and latent heats for phase changes
- Figure 1 and Table 1.
| Figure 1: p-T Perspective - Normal States: WATER (@ 1 Atm) | |||||
 | |||||
| Table 1: Phase Boundaries/Specific and Latent Heats: WATER (@ 1 Atm) | |||||
| 1 | (A) Heating or Cooling |
(B) Melting or Freezing |
(C) Heating or Cooling |
(D) Boiling or Condensing |
(E) Heating or Cooling |
| 2 |
T < 0-°C Solid (T < 32-°F) |
T = 0°C Sol and/or Liq (T = 32°F) |
0+°C < T < 100-°C Liquid (32 < T < 212°F) |
T = 100°C Liq and/or Gas (T = 212°F) |
T > 100+°C Vapor/Gas (T > 212°F) |
| 3 |
ρsol 0.92 g/cm3 (57.2 lbm/ft3) |
ρsf = ρliq - ρsol 0.08 g/cm3 (5.2 lbm/ft3) |
ρliq 1.00 g/cm3 (62.4 lbm/ft3) |
vfg = vg - vf 1679 cm3/g (26.8 ft3/lbm) |
Ideal Gas pV = m(R/M)T |
| 4 |
csol,avg 1.96 J/g°C (0.45 Btu/lbm°F) |
hsf = hf - hs 333 J/g (144 B/lbm°F) |
cliq,avg 4.2 J/g°C (1.00 Btu/lbm°F) |
hfg = hg - hf 2252 J/g (970 Btu/lbm) |
cv and cp 1.41,1.87J/g°C |
- Figure 1 is a perspective (not to scale) of the p-v-T surface for water. Locations along the dashed line indicate "normal states." Solid, liquid and gas regions, notated "(A), (C), and (E)" in Figure 1, have their single phase properties listed in Table 1 columns, "(A), (C), and (E)," respectively.
- Table 1: Row 1 presents the common names of "heat event" for single phase (A, C or E) and phase change (B or D). Relavant properties are in rows 2,3, and 4.
- Table cell (4A) and (4C) list (4)
Energy Equation: At its basis, the energy equation recognizes energy of a system and equates its potential changes to the energy change mechanisms, work and heat. We find energy equations written in three mathematical forms: Differential, Increment, and Rate.
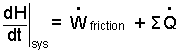 |
(1) |
4.02 Normal Properties: WATER
Here, we study events of water (as the system) subject to the condition of constant pressure at one atmosphere. The brief phase diagram for water
 (below right) indicates, as a shaded domain, the normal states of of water. Normal means "having the pressure, one atmosphere."
(below right) indicates, as a shaded domain, the normal states of of water. Normal means "having the pressure, one atmosphere."
There are very many industrial events of water that occur at constant atmospheric pressure. Once we learn these cases, extension of technique to other pressures follows without difficulty. Two common data formats for normal properties of water are presented below.
Premise presently unwritted!