| THERMO Spoken Here! ~ J. Pohl © ( C6200~8/15) | ( C6300 - 3.22 Rotational KE) |
3.21 Events: IDEAL GAS
Many gases as systems qualify as being "IDEAL" in their events because their properties pressure, temperature and specific volume conform closely to the ideal gas equation. In those states, the specific heats depend solely on temperature (are independent of pressure). How to check, using property information whether (and over what ranges) pv = (R/M)T is valid is an important and easy task but is an advanced topic. Herein substances to be modeled as IDEAL GAS are identified as such by the author. IDEAL GAS properties are distinguished by the superscript,*, which means "this gas has been evaluated and found to be an IDEAL GAS."
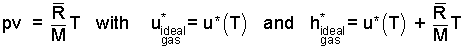
| (1) 1 |
All substances have the properties internal energy, pressure, specific volume and enthalpy. For the ideal gas, the pv of enthalpy can be expressed as (R/M)T where M is the gas molecular weight and T is the temperature (in Rankine or Kelvin degrees). Neither the internal energy nor the enthalpy of an ideal gas is measurable and they are functions of temperature. To make them quantitative, two specific heats are defined which are:
| The specific heat at constant volume, cv* ~ [E/mθ] (spoken as "c sub v") and |
| The specific heat at constant pressure, cp* ~ [E/mθ] (spoken as "c sub p"). |
Equations below show how ideal gas differences of internal energy and enthalpy are expressed in terms of specific heats and temperature differences.
dh* = c*p(T)dT du* = c*(T)vdT
Δh* = c*pΔT h*2 - h*1 = c*p(T2 - T1)
Δu* = c*vΔT u*2 - u*1 = c*v(T2 - T1)
By definition, h ≡ u + pv, consequently,
Δh* = Δu* + Δ(pv)
But for the ideal gas, Δ(pv) = (R/M)ΔT.
Hence
Δh* = Δu* + (R/M)ΔT.
Next, expressing enthalpy and internal energy in terms of specific heats, we have.
c*pΔT = c*vΔT + (R/M)ΔT
So our result is:
c*p = c*v + (R/M)
Specific heats of substances vary with temperature. The trend is that they vary the least with gases, more so with liquids and the most with solids. Sometimes, careful, second-level analysis must account for this. But for preliminary calculations it is expedient (and wise) to employ the Mean Value Theorem. The specific heat values used in this writing are estimated average values over their temperature range of application. A topic for a rainy afternoon is specific heats.
There is a very convenient rule for specification of specific heats of the monatomic and diatomic gas molecules. The energy of a gas is principally kinetic. Einstein and others studied internal energies of the simpler molecules (monatomic and diatomic structures) and deduced theoretically that the specific heat at constant volume was related directly to the "degrees of freedom" of the molecules. A monatomic gas has kinetic energy in the x, y, and z direction - 3 degrees of freedom. The energy of a diatomic molecule is kinetic energy in the three directions and rotational energy about two of the axes of the diatomic molecule - 5 degrees of freedom.
The rule from molecular theory is:
"... for an ideal gas, there is contributed to the specific heat
at constant volume, the amount, 1/2(R/M) for each degree of freedom of the molecule."
| MOLECULAR STRUCTURE |
cv | cp | γ = cp/cv |
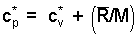 | |||
| monatomic (3 degrees of freedom) |
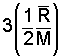 |
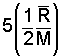 |
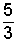 |
| diatomic (5 degrees of freedom) | 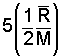 |
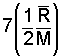 |
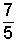 |
|
|||
Thus the specific heat at constant volume of a monatomic ideal gas, having three degrees of freedom, equals 3 [(1/2)(R/M)] or (3/2)(R/M). Diatomic molecules possess three directions of translational kinetic energy of their mass centers. But more energy might be possessed as they rotate about their centers. Three more degrees of rotation might be anticipated. There are only two. Analogous to a majorette's baton, rotation along the axis of the baton (about an axis extending through the center of the rubber end-caps) begets little energy. Diatomic molecules have 3 translational and 2 rotational degrees of freedom - 5 degrees of freedom.
Ratio of Specific Heats:: The table includes the ratio (or quotient), c*p/c*v for the ideal gas. This number is very important because it is the exponent, of a special polytropic process described, p vγ = constant. Such highly theoretical processes are entirely without friction (reversible) and without heat. Often they are called reversible-adiabatic processes. A special new property, entropy, is associated with the such processes. With no heat but work and the idealization of no friction, this model corresponds to the direct, loss-less interchange of work to gas energy and the reverse. Such events are also called isentropic. (Entropy is a topic of other texts).
For gases that are not ideal, specific heats are determined experimentally. While "c sub v" of a gas is determined by a constant-specific-volume process (or equivalent), once values are established, they can be used for all state changes of the gas - not restricted to constant-specific-volume processes. The same is true for the specific heat at constant pressure, "c sub p." A last comment: Condensed phases (liquids and solids) also have two specific heats but these are virtually identical in numerical value. So "c sub v" and "c sub p" are called "c."
Events: IDEAL GAS
Many physical gases of systems qualify as being "ideal" in their events because their properties pressure, temperature and specific volume conform to the ideal gas equation. In those states, the specific heats of gases depend solely on temperature (are independent of pressure). How to check, using property information, whether (and over what ranges) pv = (R/M)T is valid is an important and easy task but is special topic.
Premise presently unwritted!