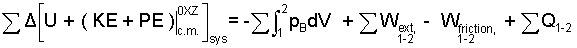| THERMO Spoken Here! ~ J. Pohl © | TOC NEXT ~ 159 |
3.19 Energy Equation I: Simple Compressible SUBSTANCE
Previous pages presented definitions and assembled an energy equation relating interactions of system energy, work and heat. The table serves to review the energy equation we have (Row 5) and how it was obtained.
Extrinsic Perspective: The extrinsic perspective with system-displacement terms (Row 1) resulted in the Extrinsic Energy Equation (Row 2). This energy equation failed, produced nonsense, when applied to bodies that experienced catastrophic crashes as did the dropped car and the train wreck. In those discussions, internal energy and heat were introduced.
Complex and Simple Substances: All thermodynamic substances have work modes, i.e., distinct manners by which work might occur. Every substance has at least "compression" as a work mode. Stubsances with one appreciable work modes are called simple, those having more than one mode are called complex.
A new, intrinsic perspective is needed to make some sense of collisions (Row 3). While there is but one intrinsic energy (internal energy), intrinsic work can occur in a number of ways which are called modes (see other texts). The "common-to-every-SUBSTANCE" intrinsic work mode is compression work (the internal energy of all SUBSTANCEs can be changed by compression). Table Row 3 presents internal energy and compression work as energy equation terms. Read the table through Row 3; then resume below the table.
| Extrinsic Energy Equation | |||
| (1) | System | Energies | Work |
| BODY (extended body) |
Kinetic and Potential (KE + PE)c.m. |
Exrinsic (force through displacement) Wext= ∫ F · dX |
|
| Extrinsic Energy Equation ΣΔ(KE + PE)c.m. = Σ Wext | |||
| Intrinsic Energy Equation | |||
| System | Energy | Work | |
| 3 | Intrinsic (e.g., a compressed gas) |
Internal (temperature and specific volume) U |
Intrinsic (pressure and volume change) - ∫ pBdV |
| 4 |
INTRINSIC ENERGY EQUATION |
ΣΔU = - Σ ∫pBdV+ ΣQ |
|
| 5 |
ENERGY EQUATION |
Σ[ΔU +Δ(KE + PE)c.m.] = -Σ∫pBdV + ΣWext + ΣQ |
|
|
• To write, for example, ΣW when there is but one W can be forgiven in recognition of the danger in writing W, which presets the mind, when in fact, ΣW is applicable. • W is a subset of ΣW. • All SUBSTANCEs have the intrinsic work mode, "compression work:" ( -∫pBdV) • Heat is necessary to make things "come out even." Some say the energy equation is the definition of heat. | |||
Analogous to the extrinsic perspective, intrinsic terms of "system energy change" are written left-of-equality and are equal to "energy transfer mechanisms." The Intrinsic Energy Equation, (Row 4) includes internal energy, compression work, and the term it defines, heat. Row (5) presents our Energy Equation as a sum of the extrinsic and intrinsic. The descriptors, extrinsic and intrinsic were necessary but never welcome "on our way" to a single energy equation. This energy equation will be transformed into its final form in Chapter 5.
This table is a preview for terms that are only slightly familiar and a glance over the shoulder at kinetic and potential energy. Nomenclature, sign conventions, etc, momentarily aside, the table displays the hierarchy of ideas and equations that began with body studies and lead to this basic tool. Our point of departure for future thermodynamic investigations is the energy equation:
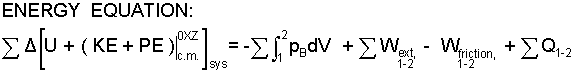
| (3) 3 |
The equation is complicated because thermodynamics is complicated. Always to begin with the above Energy Equation; it describes many physical events. It is made specific to one event by deletion of the inapplicable terms. Within one step of reduction you might arrive at one of the cases below which are presented for discussion, not for use. Use the Energy Equation; not its reductions.
Equilibration Event: System events with no work and no heat occur to systems constrained initially to have differences of energy among its energy types. Said system will stay that way (constrained) until the constraint is removed, whereupon the initial system energy re-distributes itself among its energy types subject to the constraints of the next state. Such changes are called "equilibration." For example, water flows to a new level or some ice and water become cold water. Heat and work being zero means all term right of equality are zero individually, not by event balance.
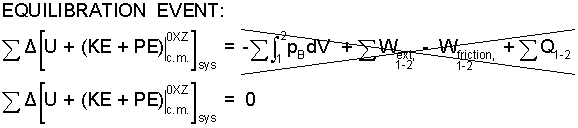
| (4) 4 |
Frictionless Event: Energy change mechanisms (Energy Equation - right of equality) are work and heat. The energy of all matter can be changed by compressing it. Compression work occurs over boundary surface, B. Pressure at the location of work, pB, is that of the surroundings. Frictionless (as used here) means pB, is assumed to be that of the system SUBSTANCE psys. No friction means system pressure is the uniform, preferred pressure of its SUBSTANCE, the pressure the SUBSTANCE would exhibit were it leisurely occupying its space despite rushing boundaries of the event.
Δ(KE + PE) associates directly with the sum of extrinsic works, right of equality. The term "sum of heats" ( ΣQ ) is a bit false. Thermo-academically it is the easiest term (we avoid it). It is dismissed as being known (stated or given), or what is unknown (the quantity to be calculated) or it is argued to equal zero.
Finally there is friction, the ever-present spoiler of plans. Friction always occurs and always results in localized increases of temperature. It is so "everywhere," to write a summation ahead of it makes no sense. Some authors included friction in the sum of heats. Since friction is so difficult to specify, we are always obliged to apply some idealization of the system event that means "friction is assumed zero."
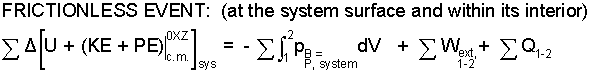
| (5) 5 |
For frictionless constant pressure events the equation reduces to the following.
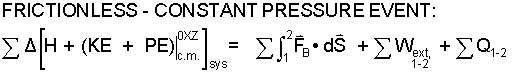
| (6) 6 |
Sign Convention of Work Many competent students upon finding thermodynamics difficult to understand apply proven learning skills to the task: extra effort, time and reading of other texts. As a rule, to read texts other than the assigned text is a good idea; thermodynamics texts can be an exception to that rule.
A principal difficulty in cross-reading thermodynamics is the sign convention authors use for work. All terms left of equality associate with system energies - there is no sign difficulty there. Terms right-of-equality are energy change mechanisms of two types: work or heat. It is with work that the simplest equation of thermodynamics appears written oppositely. There being two conventions is troublesome to students.
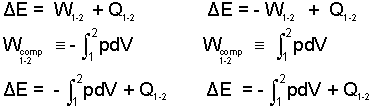
| (7) 7 |
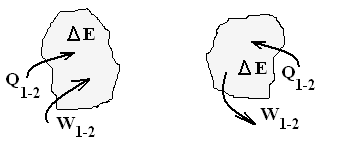
Two system symbols with "arrows" representing heat and work are drawn to the right. Beneath the images are written equations. In the left image and column (as in this writing), the simplest energy equation is written with work defined to be positive if its effect (in the absence of all other effects) is to increase system energy. By this definition work in the energy equation (W) is preceded by a positive sign in the equation. Compression Work is developed from the vantage that system volume is diminished (V2 - V1 is negative) with the piston force and displacement coincident, to conform, with the positive work definition, this integral must be preceded by a negative sign (the energy of a gas increases as its volume decreases, as it is forced into a smaller space). Both ideas combine to yield the result at the bottom of the left column of equations.
Other authors see work as positive with a decrease of system energy. In the image of "system" (the right side image) the work arrow comes out of the system. This way the work of an event is represented in the energy equation with a negative sign (the first equation in the right column). The view, Expansion work better suits this discussion. Expansion work, the second equation in the column takes a positive sign. But its integration and entry into its energy equation (with its convention) arrives at the same result - bottom equation of both column.
This means the thermodynamics of matter are the same irrespective of sign convention. Thermodynamically true, but lack of agreement among well-financed commercially dominant authors continues to foist this pedantic problem on students, fighting to learn thermodynamics. Enough already. Ancient mariners solved problems with convention: they invented "port" and "starboard." Mathematics solved the idea, "zero." Still thermo authors continue the pedantic debate, "Work is positive (tastes great), no work is negative (less-filling).
Extrinsic Energy Equation Part of the equation at the top of this section is the first step of thermodynamics, the transformation of Newton's concepts, momentum and force into energy and work.
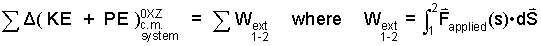
| (8) 8 |
Internal Energy Equation (as called by some) arises for use with systems composed of many particles: solids, liquids and gases. Such matter has displacements with extrinsic work and deformations with the new work perspective - intrinsic work. Compression (work) applied to a system was argued to increase the system energy - internal energy. Every system is susceptible to energy change by compression work which has been made quantitative in terms of pressure and volume change.
Friction occurs to extrinsic events but is largely ignored. Friction also occurs interior to systems as evidenced in part by temperature increase within the system. System temperatures that differ from surrounding temperatures, given time, equilibrate with the surroundings; that energy transfer being named heat. Consequently "heat" is an added term. Many texts use the simple symbol, Q, with the note below that this is "net" heat. If you write ΣQ, you will never forget the net. The sign convention is heat (or work) occurring alone that causes an increase of system energy is positive.
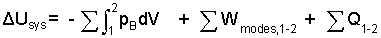
| (9) 9 |
When the equations are summed, the identity of terms is retained and the equality is respected - energy changes are "left of equality" and energy transfer mechanisms are listed "right of equality."
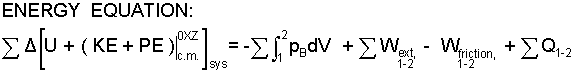
| (10) 10 |
This equation is almost an "A to Z" grocery list. Most physical events are adequately modeled using but a few of the terms. A shorter equation with the diversity of work represented lumped together as, ΣW is useful:
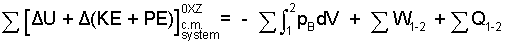
| (11) 11 |
The above equation is reasonably complete. It applies for all events including those with phase change. (We avoid phase change in this chapter). The equation form is "increment event." A rate form is presented at the end of this chapter. The summations signs are "reminders" - look around. Rotational kinetic energy has been bypassed for the sake of brevity. Friction is always present and difficult; there is some discussion of friction here.
3.18 Energy Equation: SUBSTANCE I
Previous pages presented definitions and assembled an energy equation relating interactions of system energy, work and heat. The table serves as a way to take stock of the Energy Equation we have and how it was obtained.