| THERMO Spoken Here! ~ J. Pohl © | TOC NEXT ~ 1 |
3.11 About Enthalpy
Our path is to introduce internal energy and enthalpy of a pure substance
"Pure Substance," is assumption that a substance is
pure. One molecular species and nothing else."
in the least-complicated manner, simply as properties, then to discuss how they apply to physical events or processes.
In general the properties of simple thermodynamic substances are functions of two variables.
At the least complicated level, internal energy (enthalpy) of all thermodynamic substances depend upon its pressure and temperature. To "depend upon" means the substance might exhibit a change of internal energy upon experiencing a change of either pressure or temperature, or change of both happening together.
The simplest thermodynamic substances are called a "simple compressible," meaning...
In general the properties of simple thermodynamic substances are functions of two variables. Properties are studied on a per unit mass basis. By classical choice internal energy (u) is defined to be a function of temperature and specific volume. In the very basic, constant pressure energy calculations (for which there are very many physical instances and industrial applications) the property grouping "internal energy (u) plus pressure times specific volume (pv) arises, i.e., u + pv. For the convenience it affords with constant pressure calculations and for other uses seen elsewhere, the three-property combination is joined to form a fourth property, enthalpy. Mathematical notations for internal energy, the definition of enthalpy and its math notation are:
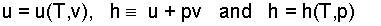
| (1) 1 |
This equation states that the specific property enthalpy, designated by the letter "h," is a dependent variable the value of which the substance itself decides for each and every pair of values of its two independent variables, pressure and temperature.
Thus when a pure substance "A" is placed in an environment with a numerical temperature, T = T* and pressure p = p*, as a consequence of its molecular structure, the substance will exhibit a numerical value of enthalpy, h* = h(T*,p*). This idea is precisely the case when the state is single phase and is extended should two phases coexist.
All of engineering and science is steeped in mathematics. Calculus of functions of two variables, such as h = h(T,p), is an extension of single variable calculus. The derivative is defined as the sum of two "partial derivatives."

| (2)2 |
The entities,
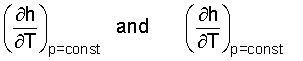
| (3) 3 |
are called partial derivatives. Now would be a good time for you to check out the above beginning ideas in a calculus book. But for our immediate needs, things become simpler, quickly. SOLID (or liquid) LIQUID INTERNAL ENERGY: The volume of a solid (or liquid) are "nearly" incompressible.
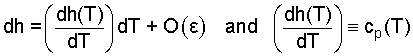
| (4) 4 |
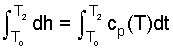
| (5)5 |
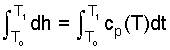
| (6) 6 |
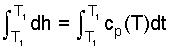
| (7) 7 |
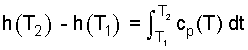
| (8) 8 |
3.12 About Enthalpy
Our path is to introduce internal energy and enthalpy in a general manner, simply as properties, then to discuss how they apply to physical events or processes.
Premise presently unwritted!