| THERMO Spoken Here! ~ J. Pohl © | TOC NEXT ~ 141 |
3.09 Compression Work
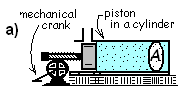
Compression work will be introduced as occurring to a gas (many bodies as a collective system) contained in a horizontal piston and cylinder apparatus which is idealized to function without leakage or friction. By action of the assumed frictionless mechanical crank and gear, the piston is advanced a differential distance into the cylinder. With the crank, piston and gas as system The force-displacement of the crank is work delivered to it. With compression of the gas, it is obvious there is work but the apparatus is arranged so the gas experiences zero kinetic energy change and zero potential energy change.
Sketch b) depicts conditions after the crank has pushed the piston slightly (and slowly) to the right, an incremental distance ΔX.
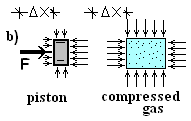
Work arrived to the piston (as system) via the crank and gear. That mechanical force and pressure-force of atmospheric air at the left face moved the piston in the direction of its displacement amounting to positive work. The initial and final velocity of the piston are zero (ΔKE = 0). Also, the piston center of mass moved horizontally (ΔPE = 0). What happened is work passed "through the frictionless piston" to the gas. The Extrinsic Energy Equation applied to the piston modeled as an extended body, confirms this:
|
[ | (1) 1 |
The summation sign is expanded; there are two works of the piston.
| 0 = Wpiston via shaft,1-2 - Wgas via piston,1-2 | (2) 2 |
That completes our consideration of the piston. Now address the contained gas as system. Positive energy as work passes to it via the shaft and piston face. A new energy equation for the gas is:
| ΔU + Δ(KE + PE)c.m. = -∫pBdV + ΣWext + ΣQ | (3) 3 |
For the compression event, the gas moved horizontally, its initial and final velocity of the center of mass were zero and the elevation of its center of mass did not change. Hence for the gas ΔKE and ΔPE are zero. Heat occurs with temperature difference. Initially for the gas, 0 = ΣQ. With compression, the gas temperature will increase. But the displacement being differentially small, we ignore it as negligible.
| ΔU =-∫pBdV | (4) 4 |
The term to the right is to be established. The gas, as system, is shown to the right in Figure b). In keeping with work being a force times a displacement the force for the gas is defined to be the pressure-force, pressure of the gas at the piston face times piston area. This force acts through a displacement. Representing compression work as an integral, we have:
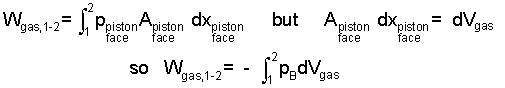
| (5) 5 |
With this final equation it is important to keep in mind that pressure, the integrand, is the pressure of the gas, "at the piston face," at the moving boundary of the gas, pB. During an actual gas compression (or expansion) pressure varies throughout the gas. Only upon the idealization "frictionless" (or reversible, some say) is pressure uniform at p throughout. Practically always we set pB equal to p but for understanding, this should be a deliberate act. A final consideration is if the energy of a gas must increase (positive work) as its volume decreases, a negative sign must precede our integral.
Compression work is a "work mode" of all substances. The internal energy of all substances can be changed by compression work. There are other work modes. Oxygen, for example, responds work-wise to magnetic force. Sensors of automotive fuel systems measure that work effect and use it in their designs.
Compression Work
 Compression work will be introduced as occurring to a gas (many bodies as a collective system) contained in a horizontal piston and cylinder apparatus which is idealized to function without leakage or friction.
By action of the assumed frictionless mechanical crank and gear, the piston is advanced a differential distance into the cylinder. With the crank, piston and gas as system The force-displacement of the crank is work delivered to it. With compression of the gas, it is obvious there is work but the apparatus is arranged so the gas experiences zero kinetic energy change and zero potential energy change.
Compression work will be introduced as occurring to a gas (many bodies as a collective system) contained in a horizontal piston and cylinder apparatus which is idealized to function without leakage or friction.
By action of the assumed frictionless mechanical crank and gear, the piston is advanced a differential distance into the cylinder. With the crank, piston and gas as system The force-displacement of the crank is work delivered to it. With compression of the gas, it is obvious there is work but the apparatus is arranged so the gas experiences zero kinetic energy change and zero potential energy change.
Premise presently unwritted!